Search site
Contact
E-mail: mda07lch@shef.ac.uk
Developmental studies
How an organism develops is important in the understanding of the mechanisms key to regenerative medicine. In the adult body there is still the possibility of redifferentiation of cells, however how to induce specific cell fates requires the study of development and the genes involved in the differentiation of cells during embryogenesis.
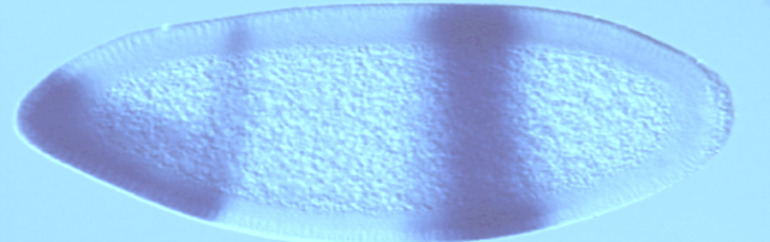
Development of the nervous system
The complex wiring of the nervous system is dependent on temporal expression of different genes leading to the diferentiation and guidence of axons. The nervous system develops from a strip of neurectoderm that folds to form the neural tube. Developmental studies revealed that the dorso-ventral patterning of the neural tube relied on a gradient of sonic hedgehog (Shh) from the floor plate and brain morphogenetic protein (BMP) from the roof plate. These gradients establish distinct neural progenitor domains which give rise to different types of neurones depending on the expression of pro-neural genes in each domain.
Gene expression is known to be under the control of chromatin modifiers including histone acetyl transferases (HATs) and histone deacetylases(HDACs) which add/ remove acetyl groups to/from chromatin respectively.
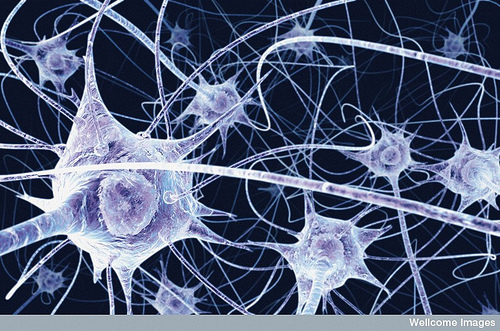 It has been shown recently that HDACs are responsible for the repression of dominant pro-mitotic genes allowing cells to express pro-neural genes and exit the cell cycle.
It has been shown recently that HDACs are responsible for the repression of dominant pro-mitotic genes allowing cells to express pro-neural genes and exit the cell cycle.
Reverse genetic studies which focused on HDAC1 knockdown in the zebrafish hindbrain lead to a massive reduction in the number of specified and differentiated motoneurones, a disruption in the patterning of remaining motoneurones and an increase in apoptosis due to non-viable undifferentiated cells.
The remaining motoneurones were suggested to be induced by the presence of a maternal source of HDAC1 during early development, showing the maintainance of motoneurone specification and differentiation depends on HDAC1.
Understanding how motoneurones are developed during embryogenesis could hold the answer to developing a treatment for diseases affecting motor control in adults such as motor-neuron disease and Machado-Joseph Disease.
Image courtesy of Flickr under creative commons license
Genetics in disease: genetic studies outside of the developmental field are also developing the understanding of disease using similar methods to those in development.
Cancer
Mouse Models:
 Mice that were especially succeptible to mammary tumour formation were found to have a retrovirus that was being transmitted through their milk (MMTV). The MMTV virus integrated into the host genome next to a proto-oncogene, Wnt-1 (homologous to drosophila Wnt) and caused this to become an oncogene. Wnt -1 studies in drosophila showed Wnt-1 to be a growth factor and overexpression of Wnt-1 caused increased cell growth and formation of tumours. Although direct overexpression of Wnt in humans has not been shown to cause cancer, alterations in the downstream Wnt signalling pathway have been associated with the formation of tumours in humans.
Mice that were especially succeptible to mammary tumour formation were found to have a retrovirus that was being transmitted through their milk (MMTV). The MMTV virus integrated into the host genome next to a proto-oncogene, Wnt-1 (homologous to drosophila Wnt) and caused this to become an oncogene. Wnt -1 studies in drosophila showed Wnt-1 to be a growth factor and overexpression of Wnt-1 caused increased cell growth and formation of tumours. Although direct overexpression of Wnt in humans has not been shown to cause cancer, alterations in the downstream Wnt signalling pathway have been associated with the formation of tumours in humans.
Image courtesy of wikimedia commons under creative commons attribution share alike 3.0 license
Zebrafish Models:
Zebrafish models have been used to identify tumour supressor genes in a forward genetics screen. Anti-phosphorylated Histone H3 is a marker for cell division and an antigen to anti-PO4 Histone H3 attached to a fluorescent marker revealed a mutations in zebrafish that lead to an increase in cell mitosis. This mutant was called 'crash and burn' and cells were shown to be stopped in M phase of cell division. Positional cloning revealed that the gene mutation was in b-Myb. Homozygous b-Myb mutantions are embryonic lethal, however heterozygous b-Myb mutants were shown to be more succeptible to tumour formation - b-Myb is a tumour supressor.
Treatment:
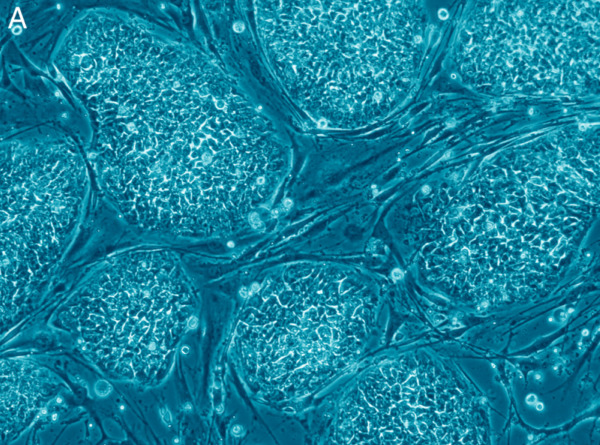 Although the above genetic studies have shown the pathways involved in tumour formation, developmental genetics leading to the understanding of cell specification and differentiation of stem cells has produced the possibility of a novel treatment for cancer. Current trials on rodents have shown that the injection of human stem cells into areas with brain tumours reduces the size of the tumour by around 80%. This shows promise for a potential new way to treat cancer in humans.
Although the above genetic studies have shown the pathways involved in tumour formation, developmental genetics leading to the understanding of cell specification and differentiation of stem cells has produced the possibility of a novel treatment for cancer. Current trials on rodents have shown that the injection of human stem cells into areas with brain tumours reduces the size of the tumour by around 80%. This shows promise for a potential new way to treat cancer in humans.
Image courtesy of wikimedia commons under creative commons attribution 2.5 license
ncetiPO4-HistoneH3
© 2009 All rights reserved.